Printable Enthalpy, Entropy, And Free Energy Of Formation Table – 2ag2o(s) ⇋ 4ag(s) +o2(g) 2 a g 2 o ( s) ⇋ 4 a g ( s) + o 2 ( g) answer what does the above problem tell us about the stability of silver in the presence of oxygen? The nist chemistry book contains: Enthalpy of combustion, enthalpy of formation, gibbs energy of formation, entropy, and heat capacity. 17.5 batteries and fuel cells;
A Fundamental View Of Enthalpyentropy Compensation (Rsc
Printable Enthalpy, Entropy, And Free Energy Of Formation Table
The standard gibbs free energy of formation of a compound is the change of gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, in their standard states (the most stable form of. Δsvap = δhvap tb = 40,657 j 373.15 k = 108.96 j/k. Calculate free energy change for a process using enthalpies of formation and the entropies for its reactants and products
This New Property Is Called The Gibbs Free Energy ( G) (Or Simply The Free Energy ), And It Is Defined In Terms Of A System’s Enthalpy And Entropy As The Following:
The energy required for vaporization offsets the increase in disorder of the system. At the normal boiling point of water, δg100°c = δh100°c − tδs100°c = 40,657 j − [(373.15 k)(108.96 j/k)] = 0 j. In this way we can build up a table of enthalpies of formation, one compound at atime,until we have afairly completetable.
Thermochemical Properties At 298.15 K:
Standard heats and free energies of formation and absolute entropies of elements and inorganic compounds. G = h − ts free energy is a state function, and at constant temperature and pressure, the standard free energy change (δg°) may be expressed as the following: 2.6 ionic and molecular compounds;
Hence There Is An Increase In The Disorder Of The System.
As in the case of the standard enthalpy of formation, we define the standard free energy of formation of any element in its allotropic form at 1 atm and 25 c example dg0 f (o 2) = 0 dg0 f (c, graphite) = 0 Define gibbs free energy, and describe its relation to spontaneity; Reaction thermochemistry data for over 8000 reactions.
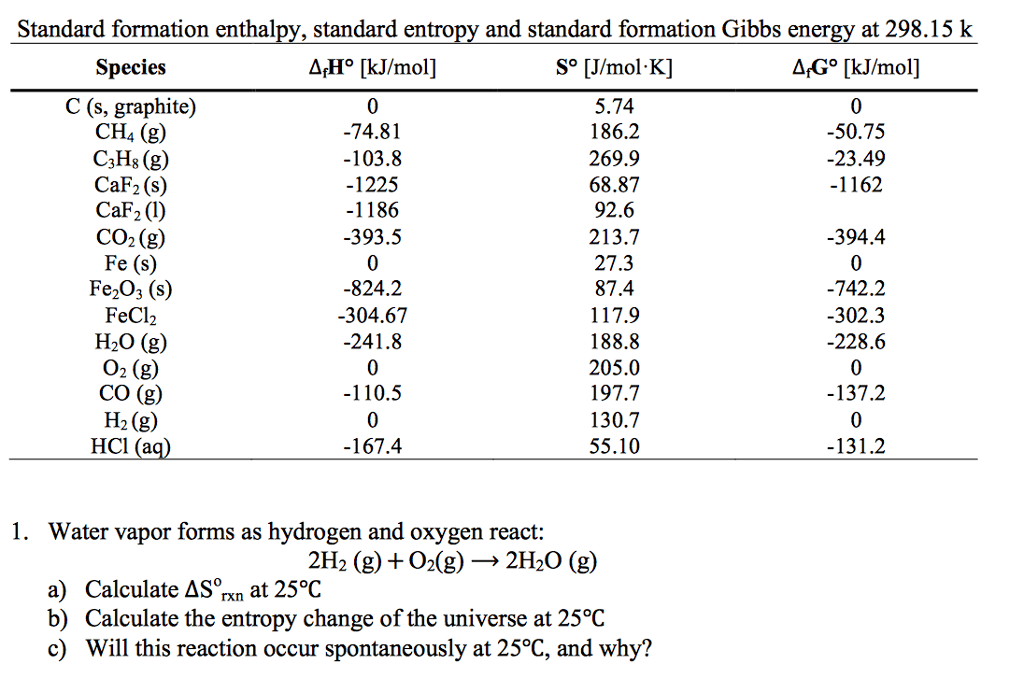
1 Using The Table Of Standard State Molar Thermodynamic Values (Table T.1), Calculate The Equilibrium Constant K For The Following Reaction At 25 O C.
Tabulated values of standard free energies of formation are used to calculate δg° for a reaction. Discuss the spontaneity of the conversion with respect to the enthalpy and entropy changes. Phase transition enthalpies and temperatures.
The Standard Enthalpy Of Formation At 25°C (298,15 K) For 1 Mol Of The Substance In Its Given State (G= Gas And L= Liquide) From Its Elements In Their Standard State (Stable Forms At 1 Bar And 25°C)
The standard free energy of formation (δg ∘ f), is the change in free energy that occurs when 1 mol of a substance in its standard state is formed from the component elements in their standard states. Wet steam, dryness fraction and enthalpy. The nist chemistry book (see link below) is an online resource that contains standard enthalpy of formation for various compounds along with the standard absolute entropy for these compounds from which the standard gibbs free.
Calculate Free Energy Change For A Process Using Free Energies Of Formation For Its Reactants And Products;
The state of the compound is specified by the following symbols: Refractive index, as a function of wavelength and temperature. If you are redistributing all or part of this book in a print format, then you must include on.
Definition And Explanation Of The Terms Standard State And Standard Enthalpy Of Formation, With Listing Of Values For Standard Enthalpy And Gibbs Free Energy Of Formation, As Well As Standard Entropy And Molar Heat Capacity, Of 370 Inorganic Compounds.
Determine the standard enthalpy change, entropy change, and free energy change for the conversion of diamond to graphite. Explain why diamond spontaneously changing into graphite is not observed. Thermochemical data for over 7000 organic and small inorganic compounds:

enthalpy, entropy and Gibbs free energy YouTube

Gibbs Free Energy and Entropy Noticias Acapulco NEWS

Solved 5. Using the data given in the table below, calculate

how to calculate gibbs free energy from enthalpy and entropy A level

Enthalpy Of Formation Of Water Water Ionizer

A fundamental view of enthalpyentropy compensation (RSC

Enthalpy, entropy and free energy YouTube

III Calculating Enthalpies STA Form IV Honors Chemistry

PPT Unit 14 Chemical Thermodynamics PowerPoint Presentation, free

enthalpy calculation worksheet

PPT Entropy, Enthalpy, and Free Energy PowerPoint Presentation, free
Enthalpy Of Formation Of Water Water Ionizer

8 Crucial Difference between Entropy and Enthalpy with Table Core

Enthalpy, Entropy, & Gibbs Free Energy YouTube

Standard enthalpy of formation and standard free energy of formation of